Company Overview
Alector (NASDAQ:ALEC), a biopharmaceutical company in clinical stage, is pioneering immuno-neurology to treat neurodegeneration by rectifying immune dysfunctions. They have identified over 100 immune targets and have three product candidates in development: latozinemab (formerly AL001), AL002, and AL101, each targeting different pathologies. Their collaboration with GSK (GSK) and AbbVie (ABBV) centers on global development and commercialization of therapies for neurodegenerative diseases. Latozinemab, designed for frontotemporal dementia (FTD), has shown promising results in early clinical trials and is currently in a pivotal Phase 3 trial, with data expected in 2025. AL101 is aimed at larger indications like Alzheimer’s and Parkinson’s disease, with encouraging early safety data. AL002, in collaboration with AbbVie, targets Alzheimer’s and is undergoing a Phase 2 trial. Alector’s approach extends to potential oncology applications, with AL009 and AL008 targeting immune checkpoint pathways in tumor evasion.
Alector pipeline (Alector 10-K)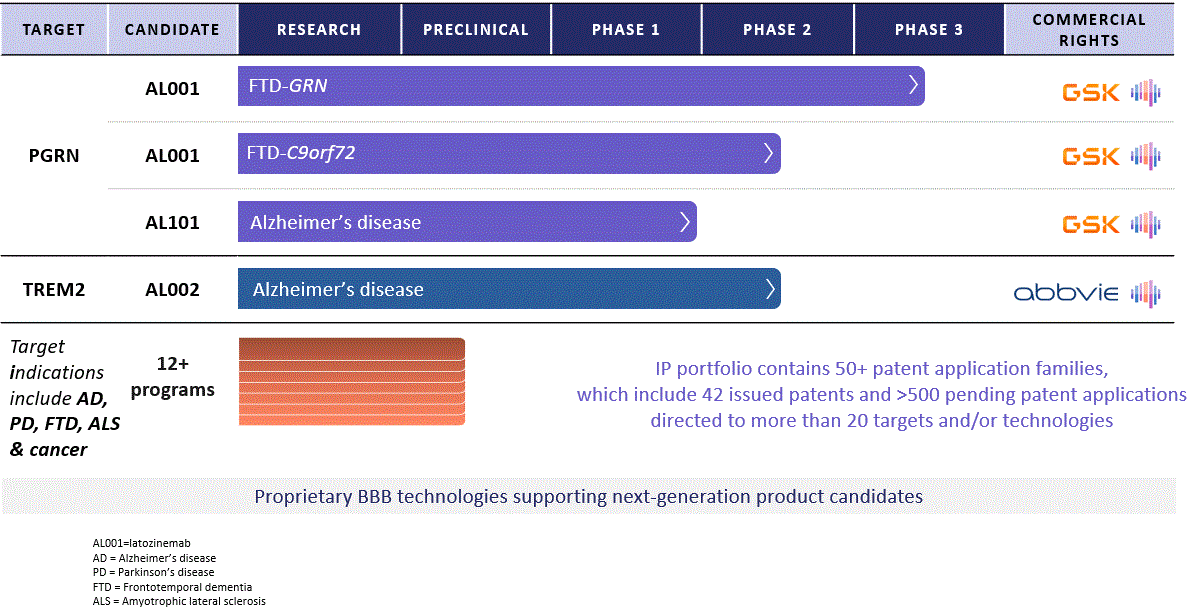
The following article focuses on Alector’s lead candidate, latozinemab.
Q1 2023 Earnings
Let’s first review the firm’s most recent quarterly earnings report. Alector reported Q1 2023 financial results with a decrease in collaboration revenue to $16.5 million from $24.5 million year-on-year due to lower revenue recognized from the GSK agreement. R&D expenses dropped slightly to $51.9 million from $53 million due to changes in latozinemab programs, while G&A expenses fell to $14.8 million from $15.6 million, primarily from reduced consulting costs. Alector saw a net loss of $45.9 million, or $0.55 per share. As of March 31, 2023, the company held $669.3 million in cash, projected to sustain operations till 2025.
Anticipated 2023 revenues range between $15-25 million, with R&D expenses of $225-245 million and G&A expenses of $60-70 million.
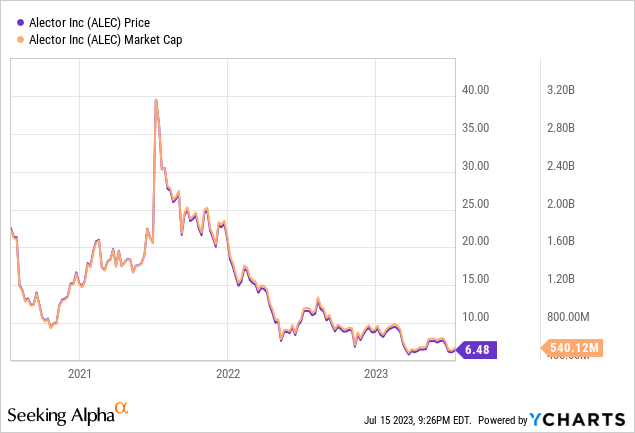
Alector’s market cap is $540.12M, with $42.18M in total debt and an enterprise value of -$87.03M.
Clinical Updates
Alector is planning to engage with regulatory bodies in mid-2023 for their pivotal Phase 3 INFRONT-3 clinical trial concerning latozinemab (AL001), aimed at treating frontotemporal dementia. The study, streamlined by emerging knowledge, is set for a data readout in early 2025, with potential for a Biologics License Application filing in late 2025. Additional data from the INFRONT-2 Phase 2 trial of latozinemab is anticipated in H2 2023, which will include results from the entire FTD-C9orf72 cohort, supporting expansion into further neurodegenerative disease indications. Their AL101 candidate is set for dose selection for a Phase 2 study on Alzheimer’s disease and Parkinson’s disease, backed by pharmacokinetic/pharmacodynamic modeling. Collaboratively with AbbVie, Alector received a $17.8 million milestone payment after enrolling the first patient for the long-term extension of the INVOKE-2 Phase 2 trial, which aims to complete enrollment by Q3 2023, with top-line data expected by Q4 2024. The company continues to invest in its research portfolio to fuel its development pipeline.
Latozinemab for Frontotemporal Dementia – Reasons for Optimism & Caution
The INFRONT-2 Phase 2 trial for Alector’s lead candidate, latozinemab, focused on the treatment of frontotemporal dementia. This open-label trial demonstrated that latozinemab was well-received by patients, successfully doubling progranulin levels from their baseline. Moreover, following latozinemab treatment, biomarkers representing lysosomal function and complement activation dropped to match levels seen in an age-matched control group. Also, the levels of Glial fibrillary acidic protein (GFAP), a prognostic marker in FTD patients, decreased significantly to match asymptomatic FTD cohorts.
However, analysts from Stifel expressed concerns a year ago over the trial’s recruitment speed, as well as the prevalence of GRN mutations in FTD patients. To date, the trial, INFRONT-3, is still “recruiting”. The analysts also raised concerns about latozinemab’s mechanism of action, focusing on SORT1 receptor, the role of which remains contentious.
In terms of the market opportunity for FTD, it’s worth noting that FTD is a severe, rapidly progressing neurodegenerative disorder that affects 50,000 to 60,000 people in the United States and approximately 110,000 people in the European Union. Currently, there are no approved therapies specifically designed to halt or slow the progression of FTD, underscoring a significant unmet medical need and market opportunity. If latozinemab proves effective and safe in late-stage trials and secures regulatory approval, it could offer a breakthrough treatment for this patient population.
My Analysis & Recommendation
In conclusion, Alector exhibits potential as a notable player in the clinical-stage biopharmaceutical domain due to its distinctive immuno-neurology approach in the treatment of neurodegenerative diseases. With a diversified portfolio that includes prime candidates like latozinemab, AL002, and AL101, and strategic partnerships with pharmaceutical bigwigs like GSK and AbbVie, the future appears promising with a multitude of potential advancements.
Yes, there are concerns about slower recruitment for trials and uncertainties related to the SORT1 receptor that have sparked questions among analysts. But I believe the strong early clinical data and the company’s innovative method of addressing immune dysfunctions reinforce Alector’s unique position in this competitive arena. Moreover, the company’s sound financial health, capable of sustaining operations until 2025 despite a net loss, speaks volumes about its financial resilience.
At a first look, the company’s negative enterprise value might seem off-putting. However, in light of the company’s creative research, significant alliances, promising portfolio, and ample cash reserves, I see this as a potential sign of an undervalued investment opportunity for the risk-tolerant investor who is ready to gamble on the inherent possibilities and risks tied to clinical-stage biotech investments.
In the short run, investors should prepare for more data from the INFRONT-2 Phase 2 trial of latozinemab expected in H2 2023, as well as potential progress on their Alzheimer’s disease contender, AL002.
My ultimate advice to investors would be to adopt a “Hold” stance, weighing the promising pipeline against imminent uncertainties and the inherent high-risk nature of clinical-stage biotech investments. While the odds of latozinemab succeeding in FTD seem slim, it appears the market has already factored this into the stock’s price. Similarly, Alector does not appear to be a suitable choice for speculation at present, although it may also not be advisable to sell due to its below-zero valuation.
Read the full article here