Vaxcyte’s (NASDAQ:PCVX) comprehensive product portfolio, combined with its recent achievements, showcases a company poised for significant growth and investor potential. With its lead candidate, VAX-24, Vaxcyte has disrupted the field of pneumococcal conjugate vaccines (PCVs) by introducing a 24-valent formulation that offers broader coverage and potential superiority over existing vaccines. By targeting additional serotypes, VAX-24 not only expands protection against invasive pneumococcal disease [IPD] but also demonstrates the company’s innovative approach to tackling emerging strains.
Furthermore, Vaxcyte’s VAX-31, a 31-valent PCV candidate, represents another breakthrough in disease prevention. With its unique serotype coverage, VAX-31 surpasses currently available vaccines, positioning Vaxcyte as a frontrunner in addressing the limitations of traditional PCVs. Additionally, Vaxcyte’s dedication to combating Group A Streptococcus infections through VAX-A1, a specialized conjugate vaccine, highlights the company’s ability to identify unmet medical needs and develop targeted solutions. This approach showcases the potential for market dominance in addressing bacterial infectious diseases.
Promising Financials
Starting with the cash position, Vaxcyte’s cash, cash equivalents, and investments stood at $957.9 million as of December 31, 2022. This is a significant increase from the previous year’s $273.1 million, with the balance including $651.6 million in net proceeds from an underwritten public offering completed in the fourth quarter of 2022. The surge in cash resources gives Vaxcyte a solid financial base to support its operations and growth initiatives.
Turning to research and development (R&D) expenses, the company reported R&D expenses of $51.6 million for the three months ended December 31, 2022, and $169.5 million for the full year 2022. These figures show a substantial increase from the same periods in 2021 when they were $23.1 million and $78.4 million, respectively. The rise can be attributed primarily to higher product and clinical development costs for Vaxcyte’s VAX-24 and VAX-31 programs. Additionally, increased numbers of R&D employees and related laboratory expenses supported these activities. Investment in research and development demonstrates commitment to advancing the pipeline, however, there are also considerable costs associated with launching new products.
Vaxcyte’s acquisition of manufacturing rights is another point of interest. The company incurred $23.0 million in acquired manufacturing rights for the three months and full year ended December 31, 2022. This was part of the upfront consideration paid by Vaxcyte in its agreement with Sutro Biopharma (STRO) during December 2022 and this strategic move should enhance the company’s production capabilities and support future progress.
Regarding general and administrative (G&A) expenses, Vaxcyte reported G&A expenses of $12.0 million for the three months ended December 31, 2022 and $39.8 million for the full year 2022. This reflects an increase from the same periods in 2021, which had G&A expenses of $6.8 million and $25.3 million, respectively. The uptick is mainly due to personnel-related costs, largely resulting from rising numbers of G&A employees needed to drive overall expansion and scaling of the business. While such expenses allow the company to function and expand, controlling them effectively is essential to ensure long-term success.
Finally, concerning net loss figures, Vaxcyte posted a net loss of $78.1 million for the three months ended December 31, 2022, and a net loss of $223.5 million for the full year 2022. Again, these numbers are larger than those over the same period in 2021 ($28.6 million for three months and $100.1 million for the full year). Net losses are not unexpected for companies in their early stages, so Vaxcyte must pay close attention to areas like expenses, revenue growth through product development, and capitalizing on market opportunities in order to achieve profitability.
Innovative Vaccine Technology
Vaxcyte is a company focused on creating innovative vaccines to prevent and combat bacterial infections. One of their primary products is VAX-24, a 24-valent pneumococcal conjugate vaccine, is under investigation to protect against invasive pneumococcal disease in both children and adults. IPD poses severe risks to infants, young children, elderly individuals, and those with compromised immune systems or chronic health issues. VAX-24 seeks to enhance current PCVs by targeting the serotypes responsible for most cases of pneumococcal disease. It employs cutting-edge synthetic methods, such as state-of-the-art chemistry and the XpressCF cell-free protein synthesis platform, to effectively generate comprehensive, high-quality vaccines.
investors.vaxcyte.com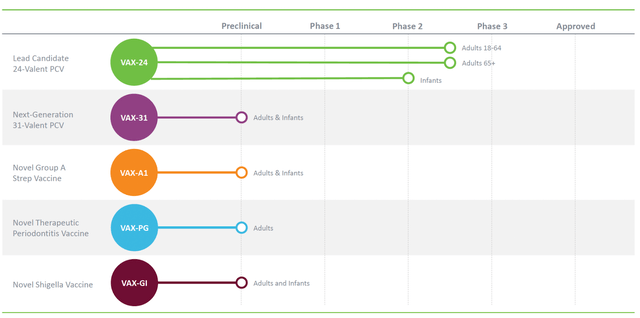
VAX-24 has reached notable milestones in its development process. The FDA granted it Breakthrough Therapy designation based on encouraging top-line outcomes from a Phase 1/2 proof-of-concept trial. In this study, VAX-24 exhibited a safety profile comparable to the standard PCV20 and met or surpassed regulatory immunogenicity criteria for all 24 serotypes. The Phase 2 trial for adults aged 65 and older is set to release top-line safety, tolerability, and immunogenicity data in the second quarter of 2023. Final outcomes, including 6-month safety data, from both Phase 2 adult trials are expected in the first half of 2023. Following the acquisition of final safety reports, regulatory engagements to guide the Phase 3 program are anticipated in the latter half of 2023. Top-line data from the critical Phase 3 non-inferiority trial in adults is projected for 2025.
Besides VAX-24, Vaxcyte is also progressing other projects in its pipeline. The VAX-24 Pediatric Program aims to develop the vaccine for infants. The company intends to submit an Investigational New Drug (IND) application and launch a Phase 2 infant trial in the first half of 2023. Top-line safety, tolerability, and immunogenicity data from this trial, following the primary 3-dose immunization series, are projected for 2025. The program includes a primary immunization series of three doses, followed by a booster dose.
Another product in Vaxcyte’s portfolio is VAX-31, previously known as VAX-XP. It is a PCV candidate covering 31 strains. The company plans to submit an IND application for VAX-31 in the latter half of 2023. Top-line safety, tolerability, and immunogenicity data from a Phase 1/2 study in adults aged 18 and older are expected in 2024.
Vaxcyte is also actively working on VAX-A1, an innovative conjugate vaccine designed to protect against infections caused by Group A Strep bacteria. The company continues to advance the development of VAX-A1, with more information about the expected timing of an IND application to be provided as the project moves forward.
Moreover, Vaxcyte has identified a final vaccine candidate for VAX-PG, a therapeutic vaccine intended to treat periodontal disease. This vaccine candidate aims to decelerate or halt periodontal disease progression. The program is still in progress, with future updates anticipated.
VAX-24: A Leader in PCVs
Vaxcyte, a vaccine development firm in the clinical phase, has reported encouraging outcomes from its Phase 2 trial of VAX-24, the leading product, in individuals aged 65 and above. VAX-24 is a comprehensive 24-valent pneumococcal conjugate vaccine candidate, designed to combat invasive pneumococcal disease. The study confirmed strong immune reactions for all 24 serotypes at all administered doses, reinforcing findings from the previous Phase 2 trial involving adults between 50 and 64 years old. The 2.2mcg dose of VAX-24, intended for Phase 3 progression, exhibited enhanced immune responses compared to the standard PCV20. Notably, VAX-24’s safety and tolerability were comparable to PCV20 across all investigated doses.
investors.vaxcyte.com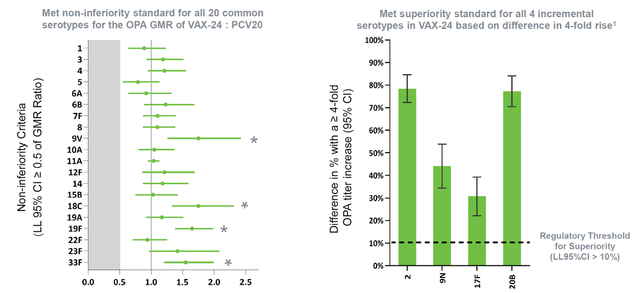
The favorable outcomes indicate VAX-24’s clinical potential in adult demographics and back the company’s objective of developing a superior PCV offering expanded coverage and improved immune responses relative to current standard-of-care vaccines. Vaxcyte seeks to engage with regulatory authorities and initiate a critical Phase 3 trial for VAX-24, anticipating top-line data in 2025. VAX-24’s success also reflects the promise of Vaxcyte’s PCV portfolio, which includes VAX-31, a 31-valent PCV candidate.
Phase 2 study data also emphasized VAX-24’s safety and tolerability, with minor-to-moderate local and systemic reactions resolving within a few days. There were no reported serious adverse events or new onset chronic illnesses linked to the vaccines under study. Coupled with the observed potent immune responses, these findings bolster confidence in VAX-24’s capacity to broaden coverage and enhance immunogenicity for IPD in adult populations.
Immunogenicity analyses from pooled data of both adult Phase 2 trials further endorsed VAX-24’s non-inferiority compared to PCV20 for shared serotypes and demonstrated elevated immune responses for several serotypes. These results substantiate Vaxcyte’s carrier-conserving strategy, implying that VAX-24 could offer an additional 10% to 28% IPD coverage for adults compared to standard-of-care PCVs.
investors.vaxcyte.com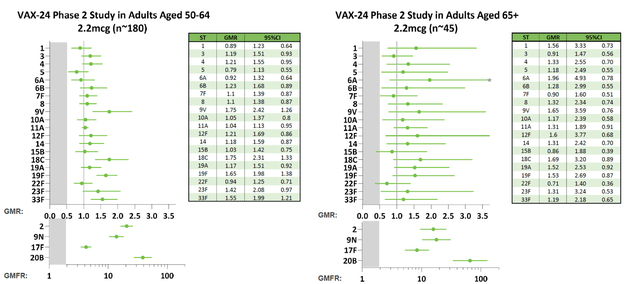
Moving forward, Vaxcyte has identified primary objectives for its PCV endeavors. The company aims to hold an End-of-Phase 2 discussion with the FDA in the latter half of 2023 to guide the Phase 3 program for VAX-24 in adults. Top-line safety, tolerability, and immunogenicity data from the Phase 3 critical non-inferiority trial in adults are anticipated in 2025. For the VAX-24 infant program, top-line data from the primary three-dose immunization series of the Phase 2 trial is expected by 2025. Additionally, Vaxcyte plans to submit an IND application for VAX-31 in the latter half of 2023 and announce top-line safety, tolerability, and immunogenicity data from a Phase 1/2 trial in adults in 2024.
Vaccine Mechanisms Pose Risks
Vaxcyte’s drugs have the potential to face certain risks and challenges in their mechanisms of action. To ensure their success, it is important to consider various factors that could impact their effectiveness and safety.
One crucial aspect is the immunogenicity and efficacy of the vaccines. While Vaxcyte aims to generate a robust immune response and provide effective protection against bacterial diseases, individual variations in immune responses could potentially affect their overall effectiveness. Factors such as age, immune status, and genetic variations may influence the immune response and potentially reduce the vaccines’ efficacy in certain individuals.
Another critical consideration is the safety and tolerability of the vaccines. Although Vaxcyte has reported favorable safety profiles in their studies, it is important to closely monitor any potential adverse events. Rare adverse events, even if unrelated to the study vaccines, can impact public perception and acceptance of the vaccines.
Furthermore, the long-term protection and durability of the immune response elicited by the vaccines should be assessed. Vaccines ideally provide long-lasting immunity, but the effectiveness can wane over time, necessitating booster doses. The durability of the immune response generated by Vaxcyte’s vaccines needs to be evaluated to determine the need for additional interventions.
The antigenic diversity of bacterial pathogens poses another challenge. Bacteria can mutate or change their surface antigens, which may render certain strains or serotypes not covered by the vaccines. Continuous monitoring of bacterial strains and potential updates to the vaccines may be necessary to ensure their ongoing effectiveness.
In addition to scientific challenges, regulatory and market considerations also play a significant role. Navigating regulatory processes and gaining approval for new vaccines is a complex and time-consuming process. Vaxcyte will need to meet regulatory requirements, demonstrate safety and efficacy, and address any concerns raised by regulatory authorities. Moreover, market competition, pricing considerations, and access to target populations can impact the commercial success of the vaccines.
Vaxcyte Is A Pioneer Compared To Competitors
In the case of VAX-24 its main competitor is Pfizer’s Prevnar 20, (PFE) a 20-valent pneumococcal conjugate vaccine. Both vaccines aim to prevent invasive pneumococcal disease, however, VAX-24 offers an advantage over Prevnar 20 by covering four additional serotypes, thus providing broader protection against pneumococcal disease. VAX-24’s Phase 2 study results in adults aged 65 and older showed comparable efficacy to Prevnar 20 for the shared serotypes and demonstrated superiority for the four additional serotypes. This scientific evidence positions VAX-24 as a superior choice in terms of serotype coverage and potential efficacy against IPD.
Regarding VAX-31, a 31-valent PCV candidate, there are currently no direct competitors with the same serotype coverage. This presents a unique advantage for Vaxcyte, as it aims to address the limitations of existing PCVs by expanding coverage to include additional serotypes. With its broader spectrum of protection, VAX-31 has the potential to provide superior immunity against pneumococcal disease compared to currently available vaccines.
investors.vaxcyte.com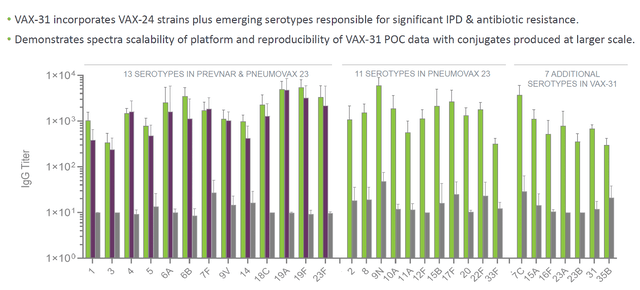
VAX-A1, Vaxcyte’s conjugate vaccine designed to prevent infections caused by Group A Streptococcus bacteria, does not currently have a direct competitor on the market. Group A Streptococcus infections can lead to a range of diseases, including strep throat and invasive infections. By developing a vaccine specifically targeting Group A Streptococcus, Vaxcyte is addressing an unmet need and has the opportunity to offer a unique solution to combat these infections. The lack of direct competitors gives Vaxcyte an advantage in terms of providing a specialized vaccine to prevent Group A Streptococcus infections.
investors.vaxcyte.com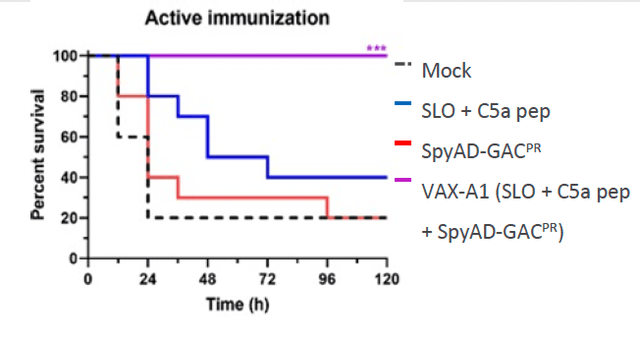
Lastly, VAX-PG, Vaxcyte’s therapeutic vaccine candidate for periodontal disease, is in a unique category with limited direct competitors. Periodontal disease, which affects the gums and supporting structures of the teeth, poses a significant health concern. Vaxcyte’s focus on developing a therapeutic vaccine to treat periodontal disease demonstrates a novel approach to addressing this condition. While other treatments for periodontal disease exist, VAX-PG offers the potential for a more targeted and immunological approach to slowing or halting disease progression.
Conclusion
In an era where global health threats loom large, Vaxcyte’s comprehensive approach to disease prevention positions the company for exponential growth. With a focus on expanding serotype coverage, improving immune responses, and addressing critical unmet needs, Vaxcyte demonstrates a keen understanding of the evolving landscape of infectious diseases. By embracing scientific rigor and staying ahead of emerging strains, Vaxcyte possesses a unique advantage in meeting the ever-changing demands of the healthcare industry.
The company’s recent accomplishments, particularly securing FDA Breakthrough Therapy Designation for VAX-24, further validate Vaxcyte’s scientific prowess and its potential to revolutionize the field of vaccine development. By leveraging cutting-edge technologies like the XpressCF cell-free protein synthesis platform, Vaxcyte has set itself apart from competitors, allowing for the efficient creation and delivery of high-fidelity vaccines with enhanced immunological benefits.
Read the full article here




